
Selecting the wrong pneumatic components for food processing can lead to contamination risks, failed inspections, and costly product recalls. With increasing regulatory scrutiny and consumer awareness, food safety has never been more critical in system design.
The most effective approach to food-grade pneumatic system selection involves understanding 3-A Sanitary Standards material requirements, analyzing CIP system pressure pulsations, and implementing proper microbial retention testing protocols to ensure complete system compliance.
When I helped a dairy processor in Wisconsin upgrade their pneumatic systems last year, they eliminated three persistent contamination points that had previously caused product quality issues. Let me share what I’ve learned about selecting proper food-grade pneumatic components.
Spis treści
- Understanding 3-A Sanitary Standards Materials
- Analyzing CIP System Pressure Pulsations
- Methods for Microbial Retention Risk Testing
- Wnioski
- FAQs About Food-Grade Pneumatic Systems
What Materials Meet 3-A Sanitary Standards for Food-Grade Pneumatic Systems?
Food-grade pneumatic systems require specific materials that meet stringent sanitary standards to ensure product safety and regulatory compliance.
According to 3-A Sanitary Standards1, food-grade pneumatic systems should use 316L stainless steel for metal components, FDA-approved2 PTFE, silicone, or EPDM for seals, and must avoid materials containing lead, cadmium, or other toxic metals that could contaminate food products.
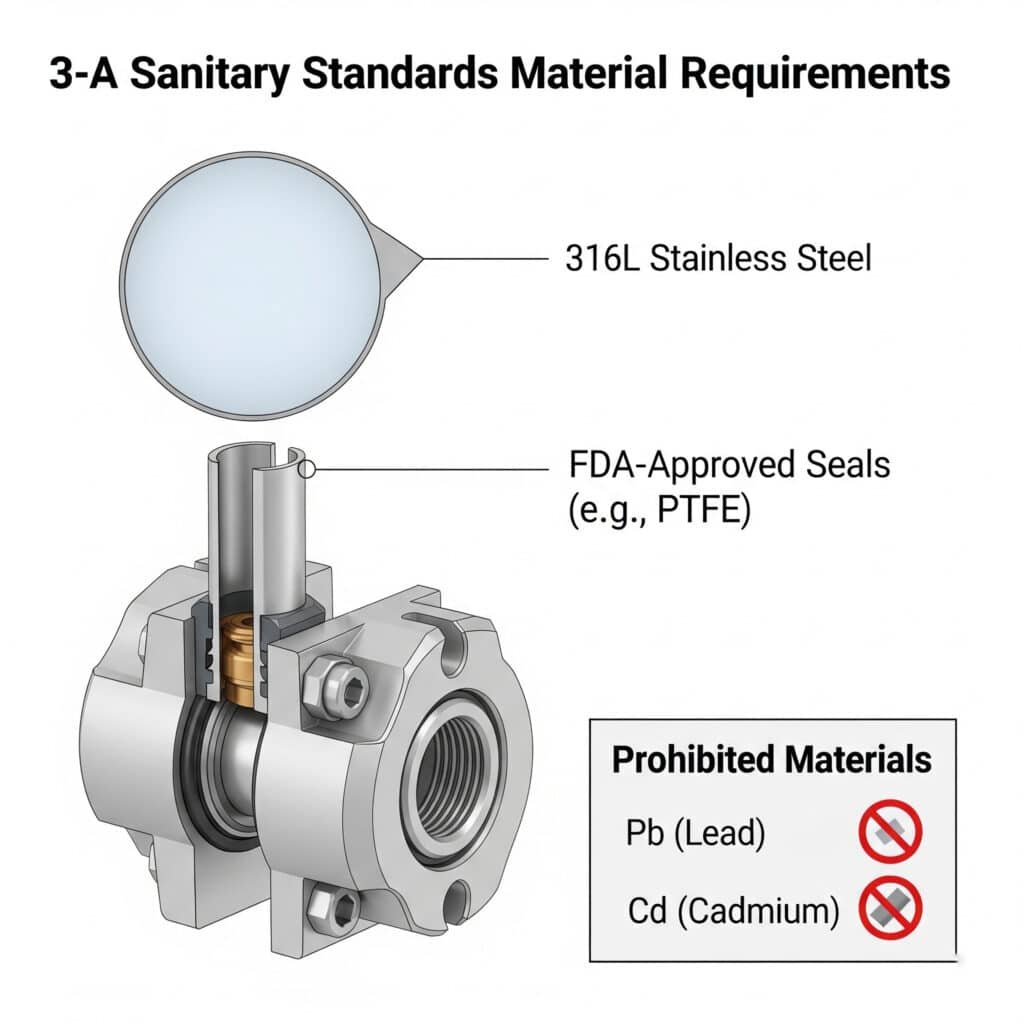
Comprehensive 3-A Compliant Materials List
Metal Components
| Component Type | Approved Materials | Surface Finish Requirements |
|---|---|---|
| Cylinder Bodies | 316L SS, 304 SS | Ra ≤ 0.8μm (32μin) |
| Fasteners | 316L SS | Ra ≤ 0.8μm (32μin) |
| Osprzęt | 316L SS, 304 SS | Ra ≤ 0.8μm (32μin) |
| Manifolds | 316L SS | Ra ≤ 0.8μm (32μin) |
Seal Materials
| Zastosowanie | Primary Materials | Zakres temperatur |
|---|---|---|
| Dynamic Seals | PTFE, UHMWPE | -20°C to 260°C |
| Static Seals | Silicone, EPDM, FKM | -40°C to 200°C |
| Gaskets | Silicone, PTFE | -40°C to 260°C |
Lubricants
All lubricants must be:
- FDA-approved (21 CFR 178.3570)
- H1 certified
- Free from mineral oils
- Non-toxic and odorless
I once worked with a beverage manufacturer who was experiencing repeated contamination issues despite using what they thought were food-grade components. Upon inspection, we discovered their pneumatic cylinders contained brass components with lead content that didn’t meet 3-A standards. After switching to proper 316L stainless steel cylinders, their contamination issues were eliminated immediately.
Material Selection Considerations
When selecting materials for food-grade pneumatic systems, consider:
- Product contact vs. non-product contact – Different standards apply based on exposure risk
- Cleaning protocols – Some materials degrade with certain cleaning chemicals
- Temperature ranges – Process and CIP temperatures affect material selection
- Certification documentation – Always maintain material certificates for audits
How Should You Analyze Pressure Pulsations in CIP Cleaning Systems?
Clean-In-Place (CIP)3 systems must deliver consistent cleaning action throughout the system, but pressure pulsations can create dead zones and reduce cleaning effectiveness.
Effective CIP pressure pulsation analysis should include flow visualization studies, pressure transducer monitoring at multiple system points, and computational fluid dynamics (CFD)4 modeling to identify potential cleaning dead zones with pulsation frequencies below 0.5 Hz.
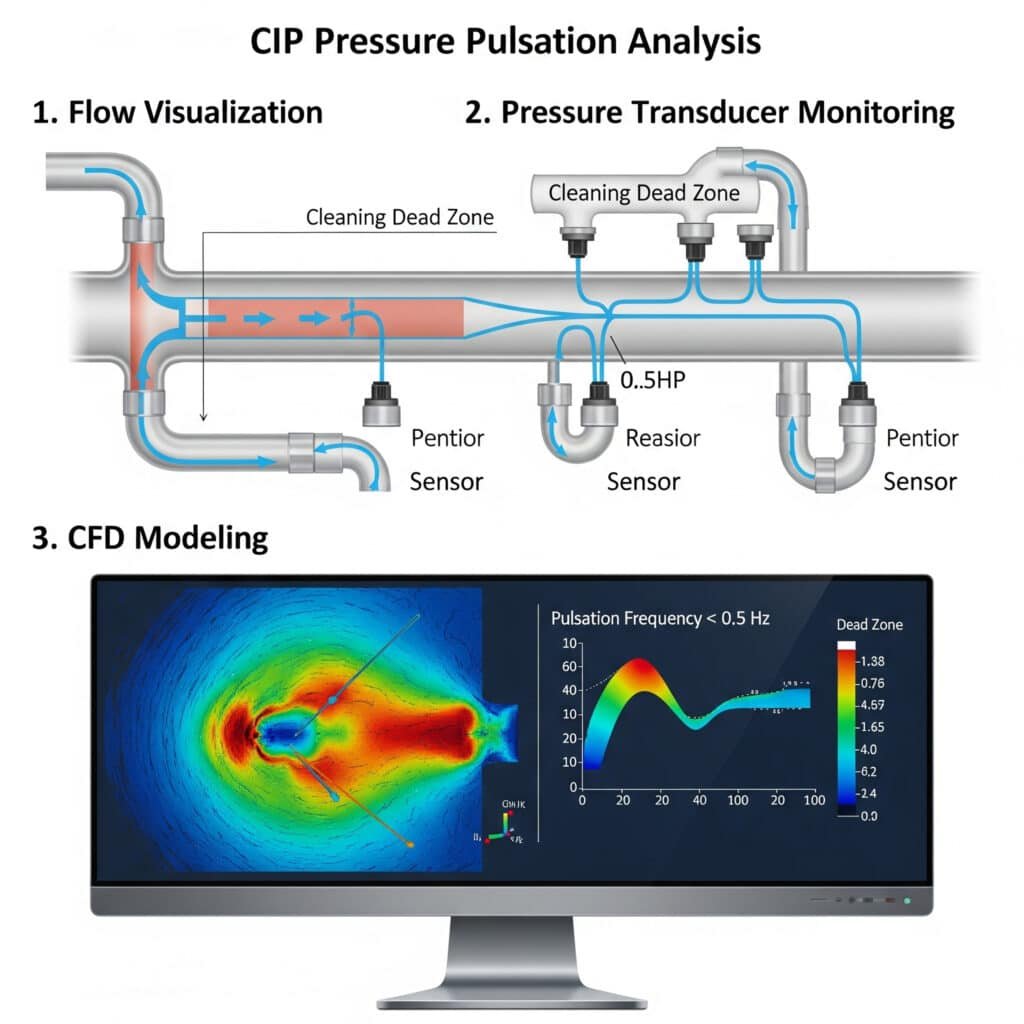
Pressure Pulsation Analysis Methods
Real-Time Monitoring
The most effective approach combines:
- High-speed pressure transducers – Minimum 100Hz sampling rate
- Flow meters at critical points – To correlate pressure and flow
- Temperature sensors – To account for viscosity changes
Data Analysis Parameters
When analyzing CIP pressure pulsation data, focus on:
| Parametr | Acceptable Range | Critical Concern |
|---|---|---|
| Pulsation Amplitude | <5% of mean pressure | >10% of mean pressure |
| Frequency | 0.5-2.0 Hz | <0.5 Hz or >2.0 Hz |
| Pressure Drop | <10% across components | >15% across components |
Optimization Strategies
Based on pulsation analysis, implement these solutions:
For High-Amplitude Pulsations
- Install pulsation dampeners near pump discharge
- Use multi-stage centrifugal pumps instead of positive displacement
- Add inline flow stabilizers
For Frequency Issues
- Adjust pump speed controls
- Modify pipe diameters at critical points
- Install resonance-breaking devices
I recently helped a cheese producer analyze their CIP system after persistent quality issues. Using pressure transducers at 12 system points, we identified significant pulsations (17% amplitude) occurring at a problematic frequency of 0.3 Hz. By installing properly sized pulsation dampeners and modifying the pipe geometry, we reduced pulsations to under 3%, dramatically improving cleaning effectiveness.
What Methods Should You Use for Microbial Retention Risk Testing?
Identifying potential microbial harborage points in pneumatic systems is critical for food safety but often overlooked in system design.
The most effective microbial retention risk testing combines riboflavin fluorescence testing under UV light, ATP swab testing5 after cleaning cycles, and high-resolution borescope inspection of internal components to identify potential harborage points.

Comprehensive Testing Protocol
Riboflavin Testing
This method provides visual confirmation of cleaning effectiveness:
- Prepare 0.2% riboflavin solution
- Circulate through system under normal operating conditions
- Drain and perform standard CIP procedure
- Inspect with UV light (365nm wavelength)
- Document any fluorescent residue
ATP Testing Strategy
| Komponent | Sampling Points | Acceptable Limit (RLU) |
|---|---|---|
| Cylinder Seals | Rod seal, cushion seal | <150 RLU |
| Valve Bodies | Spool areas, exhaust ports | <100 RLU |
| Manifolds | Internal channels, dead ends | <100 RLU |
| Osprzęt | Thread junctions, internal bores | <150 RLU |
Advanced Inspection Techniques
For thorough risk assessment:
- Borescope Inspection – Use flexible borescopes with minimum 1080p resolution
- 3D Surface Mapping – For complex internal geometries
- Hydrodynamic Flow Visualization – Using dye injection during operation
Risk Mitigation Strategies
Based on testing results, implement these solutions:
- Design Modifications – Eliminate crevices and dead ends
- Material Upgrades – Replace problematic surfaces with more cleanable materials
- Cleaning Protocol Adjustments – Modify time, temperature, chemistry, or mechanical action
During a facility audit for a baby food manufacturer, we identified critical microbial retention risks in their pneumatic transfer system using these methods. The riboflavin testing revealed cleaning solution wasn’t reaching internal components of their rodless cylinders. By switching to specially designed food-grade rodless pneumatic cylinders with self-draining features, they eliminated these harborage points completely.
Wnioski
Selecting appropriate food-grade pneumatic systems requires careful consideration of 3-A Sanitary Standards materials, thorough CIP pressure pulsation analysis, and comprehensive microbial retention risk testing to ensure product safety, regulatory compliance, and optimal system performance.
FAQs About Food-Grade Pneumatic Systems
What is the 3-A Sanitary Standards certification?
3-A Sanitary Standards is a comprehensive set of guidelines for equipment used in processing dairy and other food products. The certification ensures equipment meets strict hygienic design criteria, is constructed from food-safe materials, and can be effectively cleaned and sanitized to prevent product contamination.
How often should CIP systems be validated for food-grade pneumatic components?
Food-grade pneumatic components should undergo CIP validation at least annually, after any system modification, or when changing processed products. More frequent validation (quarterly) is recommended for high-risk products like dairy, infant formula, or ready-to-eat foods.
What are the main differences between food-grade and standard pneumatic cylinders?
Food-grade pneumatic cylinders differ from standard models by using 316L stainless steel construction (vs. aluminum or carbon steel), FDA-approved seal materials, sanitary design with minimal crevices, specialized food-grade lubricants, and surface finishes with Ra values ≤0.8μm to prevent bacterial adhesion.
Can rodless pneumatic cylinders be used in food processing applications?
Yes, specially designed food-grade rodless pneumatic cylinders can be used in food processing when they feature 316L stainless steel construction, FDA-compliant seals, self-draining designs, and appropriate surface finishes. These specialized rodless cylinders eliminate harborage points and allow complete cleaning and sanitization.
What cleaning chemicals are compatible with food-grade pneumatic systems?
Food-grade pneumatic systems are typically compatible with common sanitizers like quaternary ammonium compounds, peracetic acid, hydrogen peroxide, and chlorine-based sanitizers. However, concentration, temperature, and exposure time must be controlled to prevent damage to seals and other components. Always verify chemical compatibility with the specific materials in your system.
-
Provides a detailed overview of 3-A Sanitary Standards, Inc., an independent organization dedicated to advancing hygienic equipment design for the food, beverage, and pharmaceutical industries. ↩
-
Explains the U.S. Food and Drug Administration (FDA) regulations, specifically Title 21 of the Code of Federal Regulations (CFR), that govern materials approved for direct contact with food products. ↩
-
Describes the principles of Clean-In-Place (CIP) systems, an automated method of cleaning the interior surfaces of pipes, vessels, and process equipment without disassembly. ↩
-
Offers an explanation of Computational Fluid Dynamics (CFD), a branch of fluid mechanics that uses numerical analysis and data structures to analyze and solve problems that involve fluid flows. ↩
-
Details the science behind Adenosine Triphosphate (ATP) testing, a rapid method used to assess the cleanliness of surfaces by detecting the amount of organic matter present via a bioluminescence reaction. ↩



